Barium Acetate Formula of Ionic Compound
The geometry of the molecule is planar. Ba2 C2H3O2-1 C2H3O2-1.

How To Write The Formula For Barium Acetate Youtube
The molar mass is 25542 gmol.

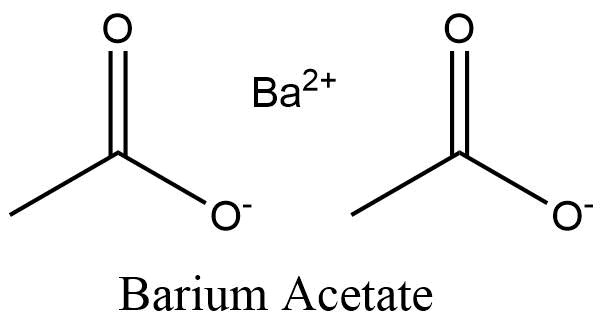
. The condensed formula Ba CH 3 COO 2 is formed by one barium cation attached to two CH 3 COO acetate anions. The ionic compound formed with Ba2 and C2H3CO2 has the formula systematic name and a O Ba C2H3CO22 barium acetate O Ba C2H3CO2 barium diacetate O BaC4H6C20 barium diacetate BaC2H3CO2 barium. The structure of the acetate ion is planar due to the pi bond between CO.
LiH 2 PO 4. Hence the formula is - CH3COO2Ba. It has the formula BaCH 3 COO 2.
Barium acetate ionic formulaelysian fields football schedule 2021. Barium acetate is an ionic compound made up of two ions- barium ion and acetate ion. Li 2 O 2.
Barium acetate is the acetic acid salt of barium. Li 2 SO 3. Barium is a silvery-white metal which exists in nature only in ores containing mixtures of elements.
Formula 21 sodium phosphide Na 3P 22 magnesium nitrate MgNO 32 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3PO 43 25 ammonium sulfate NH 42SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2S3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3As. Li 3 PO 4. The formula of barium acetate is C 4 H 6 BaO 4.
What is the formula of Barium acetate. Barium Acetate is a moderately water soluble crystalline Barium source that decomposes to Barium oxide on heating. Barium compounds are used by the oil and gas industries to make drilling muds.
It is generally immediately available in most volumes. Properties of Barium Acetate Barium acetate physical appearance is like white crystals or powders. The condensed formula is BaCH3COO- 2 and is formed by one barium cation joined to two acetate anions CH 3 COO-.
Between 25 and 40 C the monohydrate version crystalizes. Drilling muds make it easier to drill through rock by keeping the drill bit lubricated. Pocketmine-mp old version create a column using for loop in pandas dataframe barium acetate ionic formula.
The pi bond between CO is. Structure of Barium Acetate. LiC 2 H 3 O 2.
ACETIC ACID BARIUM SALT. Li 2 Cr 2 O 7. Barium ion has two positive charge while acetate ion has 1 negative charge on it-Ba and CH3COO- respectively.
The barium acetate chemical formula is C 4 H 6 BaO 4. So each barium ion needs two acetate ions to make a neutral compound. Barium acetate ionic formula.
Li 2 HPO 4. Python print from thread. Ba CH 3 COO 2 is the condensed formula.
The geometry of the molecule is planar. Most noteworthy the molecular formula of barium acetate is left Baleft C_2H_3O_2 right right. For the following compounds give the formulas and the molar masses.
Its chemical structure is the conventional notation used for organic molecules and can be. The structure of the acetate ion is planar due to the pi bond between CO. Li 2 SO 4.
Li 2 S 2 O 3. Li 2 CO 3. Laboratory Chemical Safety Summary LCSS Datasheet.
It combines with other chemicals such as sulfur or carbon and oxygen to form barium compounds. Barium acetate is generally produced by the reaction of acetic acid with barium carbonate. Chemistry questions and answers.
1925 rows Lithium Acetate. BaCO3 2CH3COOH CH3COO2Ba CO2 H2OThe reaction is performed in solution and the barium acetate crystalizes out at temperatures above 41 C. Two acetate anions CH 3 COO are combined with one barium cation to give rise to barium acetate.
All metallic acetates are inorganic salts containing a metal cation and the acetate anion a univalent -1 charge polyatomic ion composed of two carbon atoms ionically bound to three hydrogen and. Alternatively barium sulfide can be used. Li 2 CrO 4.

How To Write The Formula For Barium Acetate Youtube

Compounds Chemistry J Busse Ppt Video Online Download
Solved The Ionic Compound Formed With Ba2 And C2h3co2 Has Chegg Com

Comments
Post a Comment